Medical research safety is an essential component of advancing healthcare while ensuring the welfare of participants. As investigations into new treatments progress, the protection of patients involved in these studies becomes a top priority. Unfortunately, funding cuts in medical research can disrupt the intricate systems designed to uphold patient safety, leading to potential risks for those participating in clinical trials. Institutional Review Boards (IRBs) play a critical role in this oversight, ensuring that ethical medical studies align with established guidelines and regulations. By promoting thorough review processes, IRBs work diligently to protect research participants from harm, highlighting the profound impact funding decisions can have on the overall safety of medical research.
In the realm of healthcare innovation, ensuring the safety of individuals partaking in medical trials is paramount. Standards for participant protection, compliance with ethical guidelines, and regulatory frameworks are critical elements in overseeing human subjects research. Recent financial constraints threaten not only the viability of these processes but also the trust between researchers and the communities involved. The oversight provided by review boards, alongside safeguards for trial participants, must remain robust to foster confidence in the pursuit of medical advancements. Any disruption to the funding necessary to maintain these protective measures ultimately jeopardizes the ethical standards that underpin successful medical research.
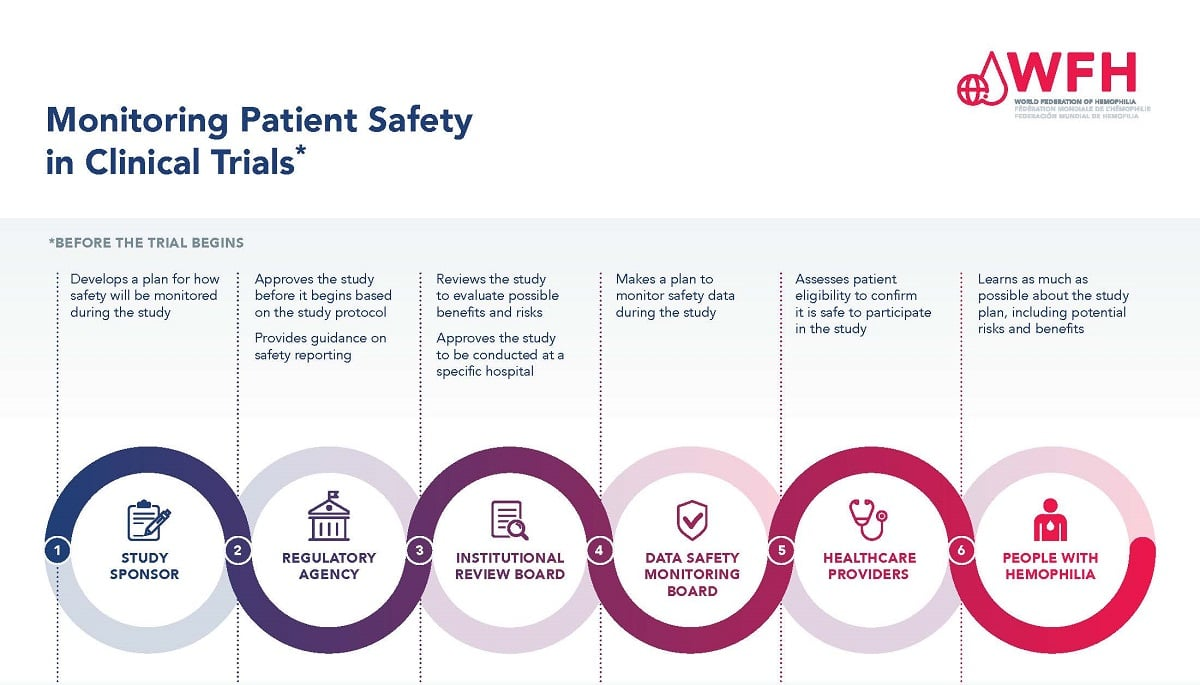
Understanding the Critical Role of Institutional Review Boards (IRBs)
Institutional Review Boards (IRBs) serve as the cornerstone of ethical oversight in medical research. They are responsible for reviewing research proposals to ensure participant rights, safety, and overall welfare are upheld throughout the study. By closely examining research design, recruitment strategies, and informed consent processes, IRBs help mitigate risks and protect individuals from potential harm. Their role is particularly vital as the landscape of medical research becomes increasingly complex, often involving multiple sites and collaborative partnerships that require a unified approach to ethical oversight.
Moreover, IRBs also monitor ongoing studies to ensure compliance with ethical standards. They assess adverse events reported during the trial and determine if there are necessary adjustments to the study protocol. This vigilant oversight ensures that researchers remain accountable and that participant safety remains a top priority. Without the guidance of IRBs, researchers may inadvertently neglect essential ethical considerations, leading to possible harm to participants and a loss of trust in the entire research process.
Impact of Funding Cuts on Patient Safety in Medical Research
The recent halt in funding for medical research has profound implications for patient safety. With vital federal resources being withdrawn, many ongoing studies face disruption or outright cancellation, profoundly affecting research participants’ rights and safety. Funding cuts often lead to understaffing and resource limitations within IRBs, hindering their ability to conduct thorough reviews and oversight of research protocols. This diminishes the effectiveness of the oversight process, potentially exposing patients to increased risks during medical studies.
Additionally, as federal grants are crucial for training and educating both IRB members and researchers, funding cuts could lead to a decrease in awareness of ethical standards and guidelines, further jeopardizing patient safety. Ethical medical studies rely on the rigorous training of professionals who can actively identify and address potential ethical issues. When funding sources dry up, it not only affects the immediate studies but also erodes the foundation of trust that participants place in the research community.
The Importance of Protecting Research Participants’ Rights
Protecting the rights of research participants is a fundamental aspect of ethical medical research. Participants must be informed about the risks, benefits, and limits of the studies they join, allowing them to make educated choices about their involvement. The role of IRBs in safeguarding these rights cannot be overstated; they are designed to ensure that every participant’s voice is heard and respected throughout the research process. This includes actively engaging with participants, addressing their concerns, and maintaining transparency regarding the scientific aims of a study and its associated risks.
In light of recent funding cuts and systemic challenges, the commitment to protecting patient rights is more critical than ever. Researchers must ensure that their studies do not compromise the trust that the community places in them. Establishing effective communication and continuous engagement with research participants can help restore faith amidst growing skepticism about clinical trials and medical research. Ethical oversight by IRBs becomes indispensable in empowering individuals to participate safely in research endeavors.
How Administrative Changes Impact Medical Research Safety
Administrative changes, such as policy shifts or leadership transitions at funding agencies, can have immediate effects on the safety of medical research. The recently enforced stop-work orders on federal grants not only disrupt ongoing research efforts but can also drastically alter the dynamics within IRBs tasked with ensuring compliance with safety protocols. These disruptions can lead to delays in the review process, causing significant setbacks for studies aimed at addressing critical health issues and protecting patient safety.
Furthermore, unsettling changes at the administrative level can foster an environment of uncertainty among researchers, potentially resulting in reluctance to engage in collaborative projects that require multiple institutional partners. When researchers are not confident in the support of their funding systems, they might hesitate to push boundaries or explore innovative solutions that could benefit patient care. Thus, the stability and consistency in funding and administrative support are essential to maintaining a robust framework that prioritizes medical research safety.
Enhancing Patient Safety through Collaborative Research Efforts
Collaborative research enables institutions to pool resources, knowledge, and expertise, thereby enhancing patient safety in medical studies. By embracing a multisite research framework, researchers can accelerate the pace of discovery while minimizing risks to participants through rigorous shared oversight practices managed by IRBs. This collaboration is especially beneficial in trials that require larger sample sizes or diverse populations to yield conclusive results.
However, when federal funding is curtailed, these collaborative efforts are jeopardized, often leaving researchers unable to initiate or expand studies critical to advancing medical knowledge. A robust system that supports collaboration not only facilitates effective oversight through shared IRB responsibilities but also helps to build a communal trust between researchers and participants. Ensuring that collaborative frameworks remain intact is essential for the future of ethical medical studies that prioritize both innovation and patient safety.
The Historical Context of Ethical Medical Research
Understanding the historical events that led to the establishment of rigorous ethical standards in medical research provides context for current practices aimed at protecting participant safety. Notable cases, such as the Tuskegee Syphilis Study, highlighted the devastating consequences of unethical research practices and led to the creation of IRBs as a means of accountability. These instances serve as potent reminders of the critical importance of informed consent, participant welfare, and adherence to ethical standards.
The evolution of ethical oversight continues to shape modern medical research practices, serving to instill confidence in participants while also guiding researchers in their responsibilities. By learning from the past, the research community can enhance its commitment to ethical principles and uphold the trust that society places in its capacity to advance healthcare while safeguarding the rights and safety of individuals involved in studies.
The Role of Ethical Guidelines in Medical Research
Ethical guidelines serve as the bedrock of medical research, providing frameworks that protect the rights and welfare of participants. Institutions and researchers must adhere to these guidelines, which dictate necessary practices for ensuring informed consent, minimizing risks, and conducting thorough reviews via IRBs. By establishing clear ethical standards, researchers can navigate complex dilemmas that may arise during study design and implementation.
Furthermore, these guidelines help foster an environment of accountability, prompting researchers and institutions to prioritize participant safety throughout the research lifecycle. Engaging in training programs that reinforce the importance of ethical practices is crucial in maintaining the integrity of medical research, ultimately leading to more trustworthy outcomes and improved public confidence in the research process.
Navigating Challenges in Collaborative Medical Research
Collaborative medical research poses unique challenges, particularly in ensuring patient safety amidst differing institutional policies and practices. When hospitals and universities partner on studies, coordination across multiple IRBs becomes crucial to maintaining consistent ethical oversight. This requires careful navigation of varying governance structures while upholding established safety standards for participants. Effective communication and shared objectives among all parties are essential to addressing these complexities.
Additionally, external factors such as funding policies and regulatory changes can add layers of difficulty to collaboration. As funding sources shift, researchers may find it increasingly difficult to align their collaborative efforts, potentially compromising the safety and efficacy of studies. To overcome these obstacles, the research community must remain adaptable, fostering relationships that prioritize ethical considerations and safeguard the interests of all research participants.
The Future of Patient Safety in Medical Research
The future of patient safety in medical research hinges not only on maintaining existing safeguards but also on evolving practices that address emerging concerns. Innovative technologies and methodologies can enhance monitoring and oversight efforts, making it essential for researchers to stay abreast of developments in the field. Continuous dialogue between researchers, IRBs, and participants will play a pivotal role in ensuring that new approaches to study design do not compromise ethical standards or participant safety.
Moreover, as societal expectations around transparency and accountability in research grow, there is an imperative for institutions to actively engage with communities and foster trust. By prioritizing ethical principles and demonstrating a commitment to participant welfare, the medical research community can pave the way for future advancements while ensuring the safety and rights of those involved in studies remain at the forefront of scientific inquiry.
Frequently Asked Questions
How does protecting research participants relate to medical research safety?
Protecting research participants is a cornerstone of medical research safety. It involves the establishment of ethical guidelines and protocols to ensure that individuals involved in a study are informed about potential risks, benefits, and their rights. Institutional Review Boards (IRBs) play a critical role in this process by reviewing research proposals to ensure participant safety and compliance with federal regulations.
What is the impact of IRB oversight on patient safety in medical research?
IRB oversight is vital for patient safety in medical research. IRBs evaluate research proposals to ensure that they are designed to minimize potential harm to participants. They assess research methodologies, recruitment strategies, and informed consent processes, ensuring that the rights and wellbeing of participants are prioritized throughout the study.
How do funding cuts affect patient safety in medical research?
Funding cuts can significantly hinder efforts to ensure patient safety in medical research. Reduced funding limits the resources available for oversight, monitoring, and ethical compliance, potentially leading to inadequate protection for research participants. Studies interrupted due to lack of funding can also undermine public trust in the research process.
What steps are taken to ensure patient safety in ethical medical studies?
To ensure patient safety in ethical medical studies, researchers must comply with stringent regulations enforced by IRBs. This includes conducting thorough risk assessments, ensuring proper informed consent procedures, and providing ongoing monitoring throughout the study to address any emerging safety concerns promptly.
What challenges do researchers face in maintaining medical research safety with reduced funding?
Researchers face numerous challenges in maintaining medical research safety amidst funding cuts, including limited resources for conducting thorough IRB reviews, reduced capability for comprehensive participant monitoring, and increased operational delays which can compromise the ethical standards of ongoing studies.
Why is IRB oversight critical for protecting research participants in medical studies?
IRB oversight is critical because it serves as the ethical framework that safeguards research participants. By evaluating study protocols and enforcing compliance with ethical guidelines, IRBs ensure that participants’ rights are upheld, including their right to informed consent and the minimization of risks, thus fostering a safer research environment.
What role does informed consent play in patient safety during medical research?
Informed consent is essential for patient safety in medical research as it ensures that participants are fully aware of the study’s purpose, procedures, risks, and benefits. This process empowers them to make an educated decision about their participation, thus promoting ethical standards and protecting their welfare.
How do historical events influence current medical research safety standards?
Historical events, such as unethical experiments in the past, have shaped current medical research safety standards by highlighting the necessity for robust ethical guidelines and oversight mechanisms, such as IRBs. These events serve as reminders of the potential risks involved in research, leading to stricter regulations aimed at protecting participants.
What can be done to improve medical research safety amid funding challenges?
To improve medical research safety amid funding challenges, institutions can advocate for increased public and private funding, enhance community engagement to build trust, and streamline research processes to maximize efficiency without compromising ethical standards. Continuous training for researchers on ethical compliance and patient safety is also crucial.
How does effective communication contribute to patient safety in medical research?
Effective communication contributes to patient safety in medical research by ensuring that participants understand study protocols, their rights, and potential risks. Open channels of communication allow participants to voice concerns and ask questions, fostering a transparent environment that prioritizes their safety and well-being.
| Aspect | Details |
|---|---|
| Funding Impact | The Trump administration’s freeze of over $2 billion in research grants has disrupted safety oversight in medical research. |
| IRB Role | Institutional Review Boards (IRBs) are essential for reviewing research proposals to ensure participant safety and ethical compliance. |
| Historical Context | Past unethical medical practices highlight the need for rigorous ethical oversight and informed consent in medical research. |
| Current Crisis | Funding cuts lead to halted studies, damaging public trust and risking participant safety. |
| Future Implications | Ongoing support is needed to maintain safety standards and continue collaborative research efforts. |
Summary
Medical research safety is critically reliant on adequate funding to ensure participant protection and ethical oversight. Recent funding cuts have jeopardized this safety, affecting the ability of institutions to conduct necessary reviews and assessments required to protect human subjects during research studies. The halt in federal research funding has already disrupted collaborative oversight efforts, leading to a concerning decline in public trust and the safety of those involved in medical trials. Continuous support and investment are essential to safeguard the rights and welfare of research participants and to promote the integrity of scientific research.