TIM-3 therapy for Alzheimer’s is heralding a new frontier in the battle against this devastating neurodegenerative disease. Recent research highlights the potential of targeting the TIM-3 immune checkpoint to enhance cognitive functions and facilitate plaque clearance in Alzheimer’s patients. This therapy works by inhibiting TIM-3, thereby unleashing the power of microglia—brain immune cells—to combat the build-up of toxic amyloid beta plaques. As a significant genetic risk factor for late-onset Alzheimer’s, the TIM-3 gene plays a crucial role in the disease’s progression, making it a promising target for therapeutic interventions. With promising results from mouse models, TIM-3 therapy could pave the way for breakthroughs in cognitive improvement in Alzheimer’s treatment plans.
The exploration of TIM-3 as a therapeutic target represents an innovative approach in addressing Alzheimer’s disease. By focusing on immune system pathways, particularly the immune checkpoint mechanisms that regulate inflammatory responses in the brain, researchers are uncovering novel strategies to enhance plaque removal. This therapeutic avenue aims to rejuvenate the brain’s natural defenses, potentially rectifying cognitive decline associated with Alzheimer’s. By examining the influence of the TIM-3 gene on cognitive impairments, scientists are poised to transform how we understand and treat this complex condition. As such, advancements in TIM-3 therapies hold the promise of delivering more effective routes for improving mental faculties in patients suffering from this debilitating illness.
The Role of TIM-3 in Alzheimer’s Disease
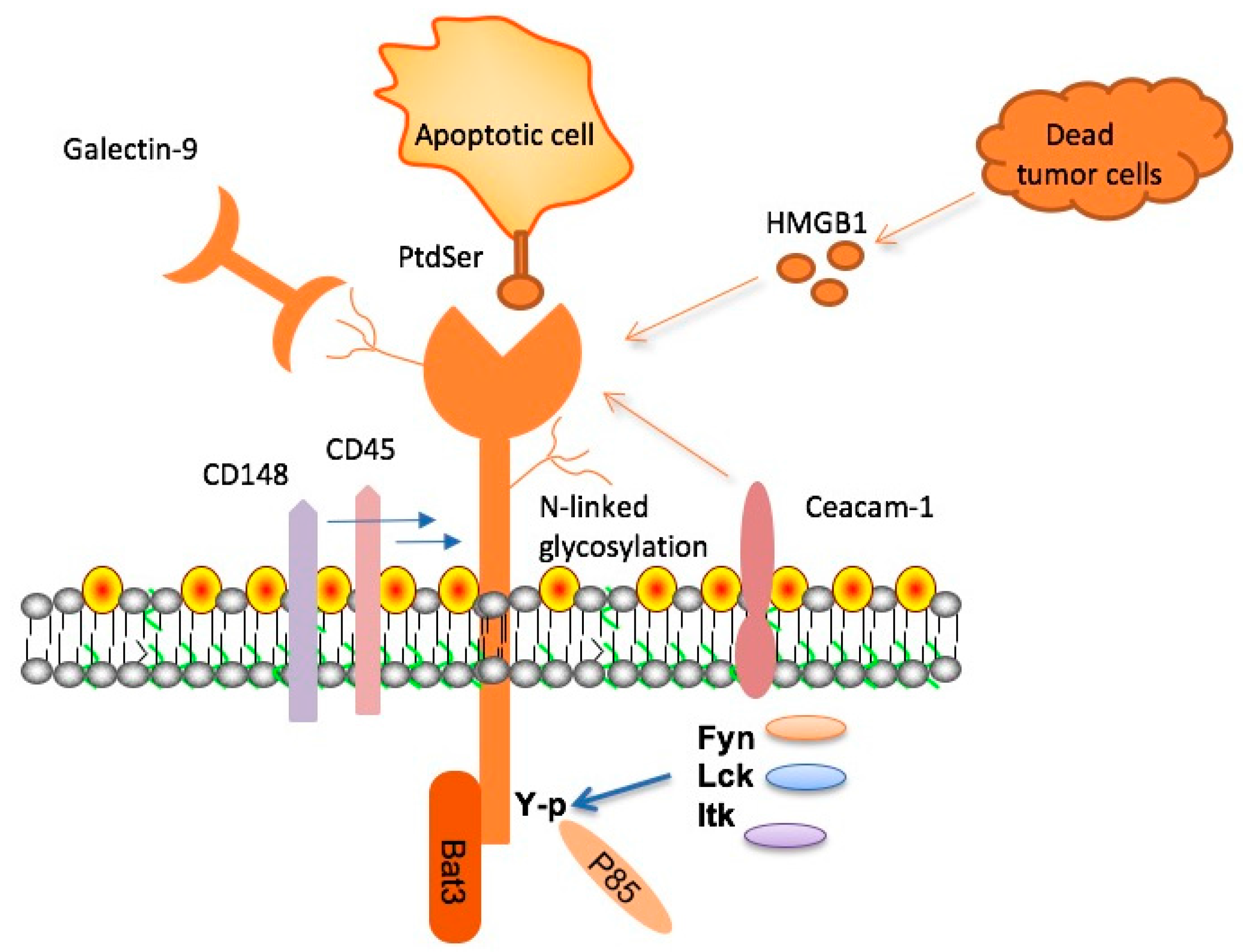
TIM-3 plays a pivotal role in the pathology of Alzheimer’s disease (AD) by acting as an immune checkpoint molecule that inhibits the activity of microglia, the brain’s primary immune cells. In Alzheimer’s, the expression of TIM-3 is found to be significantly heightened, particularly in individuals with the HAVCR2 gene polymorphism, increasing the vulnerability of microglia to become dysfunctional. This checkpoint mechanism, while initially protective, ultimately hinders the clearance of amyloid beta plaques, which are intertwined with the progression of cognitive decline in Alzheimer’s patients. As a result, the presence of TIM-3 not only complicates the immune response but also perpetuates the cycle of neurodegeneration.
Research has shown that the deletion of the TIM-3 gene in experimental models leads to enhanced activity of microglia, allowing them to resume their plaque-clearing functions. This highlights the potential of targeting TIM-3 to alleviate the burden of plaques in Alzheimer’s, thereby improving cognitive function. As microglia regain their ability to phagocytose harmful amyloid plaques, there is a corresponding restoration of memory faculties, demonstrating the therapeutic promise of TIM-3 modulation in Alzheimer’s treatment strategies.
TIM-3 Therapy: A Potential Breakthrough for Alzheimer’s
The exploration of TIM-3 therapy for Alzheimer’s disease marks a groundbreaking approach in neurodegenerative research. By utilizing anti-TIM-3 antibodies or small molecules that block the action of this immune checkpoint, there is considerable potential to rejuvenate microglial activity, effectively facilitating the removal of toxic plaques in the brains of Alzheimer’s patients. Current treatments targeting amyloid beta have yielded limited successes due to complications such as vascular damage, suggesting that TIM-3 therapy could offer a more precise mechanism of action that selectively overlays the existing pathologies present in Alzheimer’s without adversely impacting blood vessels.
The clinical development of TIM-3 therapies not only aims to enhance plaque clearance but also to improve overall cognitive outcomes in patients with late-onset Alzheimer’s. Preclinical studies using mouse models have indicated promising results, with enhanced cognitive performance observed following TIM-3 inhibition. These advancements signal hope for effective therapeutic interventions that prioritize the restoration of cognitive function, paving the way toward a future where Alzheimer’s can be better managed and potentially mitigated through targeted immune system strategies.
Understanding Immune Checkpoints in Alzheimer’s Therapy
Immune checkpoints, such as TIM-3, serve a dual role in the body’s immune regulation. While essential for preventing autoimmunity during normal immune responses, their heightened expression in neurodegenerative conditions like Alzheimer’s can lead to detrimental effects by shielding disease processes from immune attack. This phenomenon underscores the complexity of treating Alzheimer’s using immune checkpoint inhibitors. Conventional checkpoint blockers developed for cancer treatments are now being adapted for Alzheimer’s therapy, leveraging their ability to reinvigorate immune functions against amyloid plaques without provoking excessive immune reactions.
In this light, it is crucial to balance the immune response while targeting TIM-3, ensuring that while we aim to enhance plaque clearance, we do not inadvertently cause immune dysregulation. Ongoing research focuses on characterizing the precise mechanisms by which TIM-3 modulates microglial activity and defines the therapeutic window for TIM-3 inhibition, ultimately striving to create treatments that harness the protective benefits of immune checkpoints while minimizing their adverse effects in the context of Alzheimer’s-related pathology.
Recent Advances in Immunotherapy for Alzheimer’s
Recent innovations in immunotherapy have sparked renewed hope in the fight against Alzheimer’s disease. Advances in understanding the immune system’s interaction with neurodegeneration have led to groundbreaking research on checkpoint inhibitors like TIM-3, which is gaining traction as a viable therapeutic target. Unlike traditional methods targeting amyloid plaques directly, the focus on immune modulation offers a fresh perspective on treatment modalities that can potentially alter the disease’s trajectory by enhancing the body’s natural ability to respond to accumulating neurotoxic proteins.
Moreover, collaborations between research institutions, such as Harvard Medical School and Brigham and Women’s Hospital, are accelerating the discovery of immune-centered therapies. By investigating the genetic underpinnings of TIM-3 and its polymorphic variations, researchers are identifying patient-specific profiles that may better predict treatment response. This personalized approach aims to optimize immunotherapy outcomes, ensuring that therapeutic interventions are suited to the individual’s genetic landscape, thus enhancing the efficiency of plaque clearance mechanisms and cognitive preservation.
The Genetic Landscape of TIM-3 and Alzheimer’s
The genetic factors influencing the expression of TIM-3 are crucial in understanding its role in Alzheimer’s disease. Specific polymorphisms in the HAVCR2 gene, which encodes TIM-3, have been linked to an elevated risk of developing late-onset Alzheimer’s. These genetic variations may facilitate the overexpression of TIM-3 on microglia, rendering them less effective at clearing amyloid plaques. Identifying these genetic markers not only aids in the understanding of Alzheimer’s pathology but also in the development of tailored treatment strategies that address the immune checkpoint’s dysregulation.
As genetic research continues to unravel the complexities surrounding TIM-3 and its interaction with Alzheimer’s, the pathway towards personalized medicine in neurodegenerative diseases becomes clearer. By leveraging genetic insights, researchers can target specific pathways disrupted by TIM-3 in different populations, providing a clear rationale for developing gene-targeted therapies that may improve cognitive function by enhancing microglial function in clearing harmful plaques.
Cognitive Improvement Through TIM-3 Modulation
Cognitive decline in Alzheimer’s patients is closely linked to the accumulation of amyloid beta plaques in the brain, which disrupts neuronal signaling and memory retention. Research indicates that modulating TIM-3 expression can lead to significant cognitive improvements in preclinical models. By enhancing microglial activity through TIM-3 inhibitors, it becomes possible to reduce plaque load and restore cognitive functions, leading to a potential breakthrough in Alzheimer’s management. This underscores a paradigm shift from solely addressing symptoms to targeting underlying pathophysiological mechanisms.
Additionally, animal studies have demonstrated that eliminating TIM-3 from microglia leads to both reduced plaque formation and improved memory performance. Mice lacking this inhibitory checkpoint exhibited better navigation skills in complex memory tasks, signifying that TIM-3 modulation may support cognitive health even in advanced stages of the disease. As research progresses, the focus on cognitive outcomes linked to immune checkpoint modulation presents a promising horizon for future Alzheimer’s therapies.
Challenges and Future Directions for TIM-3 Therapies
While the potential of TIM-3 therapies for Alzheimer’s is promising, several challenges need addressing before clinical application. The nuanced interplay between immune checkpoint modulation and the brain’s delicate ecosystem requires careful consideration to avoid unintended inflammatory responses that might exacerbate neurodegeneration. Understanding the specific timing and context of TIM-3 inhibition will be critical in designing effective treatments that harness its beneficial effects while mitigating risks.
Future research should focus on clinical trials assessing the safety and efficacy of anti-TIM-3 therapies in human patients. Building on animal model findings, investigators will need to establish standardized protocols to ensure that treatments not only improve plaque clearance but also promote lasting cognitive benefits. As the landscape of Alzheimer’s research evolves, the integration of TIM-3 therapy could represent a landmark advance, reshaping therapeutic strategies and providing new avenues to tackle this complex disease.
Implications of TIM-3 Research for Alzheimer’s Prevention
The implications of TIM-3 research extend beyond therapeutics, offering insights into strategies for preventing Alzheimer’s disease. Understanding how TIM-3 influences microglial behavior can inform early intervention approaches aimed at halting disease progression before significant neurodegeneration occurs. By targeting the immune response early in the disease process, it may be possible to delay or prevent the onset of cognitive symptoms associated with Alzheimer’s.
Preventive strategies grounded in TIM-3 research could revolutionize public health efforts against Alzheimer’s disease. By promoting lifestyle interventions that support brain health and enhance immune functions, as well as exploring pharmacological agents targeting TIM-3, there is promise for strategies that not only treat but also proactively manage Alzheimer’s risk. As ongoing studies continue to expand our understanding, the integration of immunological insights will be key to developing effective preventive measures against this debilitating condition.
The Intersection of Cancer and Alzheimer’s Research
Interestingly, the exploration of TIM-3 as an immune checkpoint has emerged from cancer research, highlighting an intriguing intersection between oncology and neurodegenerative diseases. The strategies that have been developed to deactivate TIM-3 in the context of tumor immunity are being repurposed to address the challenges posed by Alzheimer’s disease. This cross-disciplinary approach opens new avenues for innovation in treatment strategies that can effectively target multiple pathways affected by both cancer and neurodegeneration.
By adapting cancer immunotherapy principles to tackle Alzheimer’s, researchers can leverage existing molecular insights and therapeutic frameworks to expedite developments in Alzheimer’s treatments. This collaboration also highlights the importance of interdisciplinary research in overcoming the complexities of disparate diseases, suggesting that the therapeutic promise of TIM-3 modulation could extend far beyond Alzheimer’s into other areas of neurodegeneration and age-related cognitive decline.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s disease and how does it work?
TIM-3 therapy for Alzheimer’s disease targets the TIM-3 checkpoint molecule, which inhibits microglia from effectively attacking amyloid plaques in the brain. By blocking TIM-3’s function via antibodies or small molecules, TIM-3 therapy aims to restore microglial activity and enhance plaque clearance, potentially improving cognitive function in Alzheimer’s patients.
How is TIM-3 linked to immune checkpoint regulation in Alzheimer’s disease?
In Alzheimer’s disease, the TIM-3 molecule acts as an immune checkpoint that prevents microglia from clearing harmful amyloid plaques. By inhibiting TIM-3 expression on microglial cells, researchers aim to enhance their ability to remove plaques, thereby tackling a key contributor to cognitive decline in Alzheimer’s.
What role does the TIM-3 gene play in Alzheimer’s disease susceptibility?
The TIM-3 gene (HAVCR2) has been identified as a genetic risk factor for late-onset Alzheimer’s disease. Variants of this gene may lead to increased TIM-3 expression on microglia, which hinders their ability to clear amyloid plaques, thereby contributing to the progression of Alzheimer’s.
How does TIM-3 therapy improve cognitive function in Alzheimer’s models?
By genetically deleting TIM-3 or using TIM-3 inhibitors, studies have shown enhanced plaque clearance by microglia, leading to changes in plaque structure and quantity. This therapeutic approach has resulted in improved cognitive abilities in mouse models of Alzheimer’s, suggesting a potential pathway to restore memory function in humans.
What are the potential benefits of targeting TIM-3 in Alzheimer’s treatments?
Targeting TIM-3 in Alzheimer’s treatments could provide a novel approach to improve cognitive function by enhancing the brain’s own immune response against amyloid plaques. This could address limitations of current therapies that focus solely on amyloid beta clearance.
Are there any existing TIM-3 inhibitors that can be used for Alzheimer’s therapy?
Yes, existing anti-TIM-3 antibodies, which have been validated in cancer treatments, can be repurposed for Alzheimer’s therapy. Researchers are exploring these antibodies to facilitate the clearance of amyloid plaques in the brain, potentially leading to cognitive improvement in Alzheimer’s patients.
What comparative advantages does TIM-3 therapy have over traditional Alzheimer’s treatments?
TIM-3 therapy for Alzheimer’s may offer significant advantages over traditional treatments by specifically targeting microglial plaque clearance without the vascular complications seen with anti-amyloid therapies. This specificity could lead to more effective and safer treatment options for Alzheimer’s disease.
How do researchers measure the effectiveness of TIM-3 therapy in Alzheimer’s models?
Effectiveness of TIM-3 therapy in Alzheimer’s models is evaluated using behavioral tests that assess memory and navigation abilities, as well as histological analyses to quantify amyloid plaque levels and changes in microglial activity.
Is TIM-3 therapy currently undergoing clinical trials for Alzheimer’s patients?
While TIM-3 therapy is not yet in clinical trials for Alzheimer’s patients, ongoing research is focused on testing humanized anti-TIM-3 antibodies in Alzheimer’s mouse models. Future clinical studies may evaluate its efficacy and safety in human subjects.
What are the next steps in TIM-3 research for Alzheimer’s disease?
Next steps in TIM-3 research include testing human anti-TIM-3 antibodies in mouse models of Alzheimer’s that replicate human disease more accurately. This research aims to establish the therapeutic potential of TIM-3 inhibition in preventing or slowing Alzheimer’s progression.
| Key Points | Details |
|---|---|
| Research Significance | A study suggests TIM-3 therapy could be effective for Alzheimer’s by using immune system strategies from cancer treatment. |
| Mechanism of TIM-3 | TIM-3 is an inhibitory molecule that prevents microglia from clearing amyloid plaques in the brain. |
| Impact on Microglia | Deleting TIM-3 allows microglia to attack and clear plaques, potentially improving memory. |
| Research Findings | Mice without the TIM-3 gene demonstrated better cognitive function and plaque clearance. |
| Applications for Humans | Potential human therapies may involve anti-TIM-3 antibodies to enhance plaque clearance. |
| Future Directions | Experiments are ongoing to test human anti-TIM-3 in Alzheimer’s disease models. |
Summary
TIM-3 therapy for Alzheimer’s shows promising potential in enhancing cognitive function and plaque clearance. By targeting the TIM-3 checkpoint molecule, researchers have discovered a way to invigorate the brain’s immune cells, microglia, that are essential for clearing amyloid plaques linked to Alzheimer’s disease. The recent study highlights how removing the TIM-3 expression in animal models led to improved memory and cognitive capabilities. This innovative approach may pave the way for new therapeutic strategies that effectively tackle the debilitating effects of Alzheimer’s, providing hope for patients and families affected by this devastating condition.