Prion disease treatment is entering an exciting new era, thanks to groundbreaking research that may translate into life-saving therapies. A team of dedicated scientists, including patient-scientist Sonia Vallabh and her husband Eric Minikel, are pioneers in exploring gene editing therapy for this rare but devastating group of disorders. Their recent work published in Nature Medicine indicates that by modifying a single base in the prion protein gene, they can dramatically reduce toxic protein levels in mouse models of prion disease, leading to significant increases in lifespan. As they navigate the complex pathway towards clinical trials, the hope for effective interventions against conditions like fatal familial insomnia is becoming more tangible. These developments not only signify a potential breakthrough in prion disease treatment, but they also underscore the emotional commitment of the researchers who are intimately connected to the stakes involved.
Introducing the revolutionary approaches in managing prion-related conditions, recent advancements in the field have brought fresh optimism. Known for their insidious nature and fatal outcomes, these rare disorders necessitate innovative solutions beyond conventional medical therapies. Researchers are now focusing on transformative methods such as genetic modification to combat the effects of misfolded proteins. The journey from laboratory discoveries to real-world applications is a pressing priority, especially for patients grappling with the realities of illnesses like Creutzfeldt-Jakob disease and fatal familial insomnia. Understanding these developments reshapes our perspective on neurodegenerative diseases, emphasizing the crucial nature of clinical trials and the promise of novel treatment horizons.
The Promise of Gene Editing Therapy in Prion Disease Treatment
Gene editing therapy has emerged as a beacon of hope for individuals affected by prion diseases, a group of rare neurodegenerative disorders characterized by misfolded proteins. Recent advancements in gene-editing techniques, specifically base editing, show remarkable potential in altering the genetic makeup responsible for these conditions. Researchers at the Broad Institute of MIT and Harvard have demonstrated the ability to modify a single base pair in the prion protein gene, effectively halting the production of harmful proteins. This innovative approach not only reduces the prevalence of toxic proteins in laboratory mice but also significantly extends their lifespans. Such breakthroughs underscore the critical role gene editing therapy could play in the future of prion disease treatment, providing a glimmer of hope for better management or even a cure for affected patients.
As researchers continue to refine gene editing therapies, the prospect of moving toward human clinical trials looms on the horizon. The transition from basic research to clinical application is complex and fraught with challenges; however, the progress made in preclinical settings provides a strong foundation for future investigations. For instance, using a mouse model of prion disease allowed scientists to gather crucial data on the efficacy and safety of this novel treatment. Insights gained from these animal studies will be invaluable as we prepare for potential human trials, illustrating the essential connection between laboratory research and clinical application in the quest for viable prion disease treatments.
Understanding Prion Diseases: Types and Mechanisms
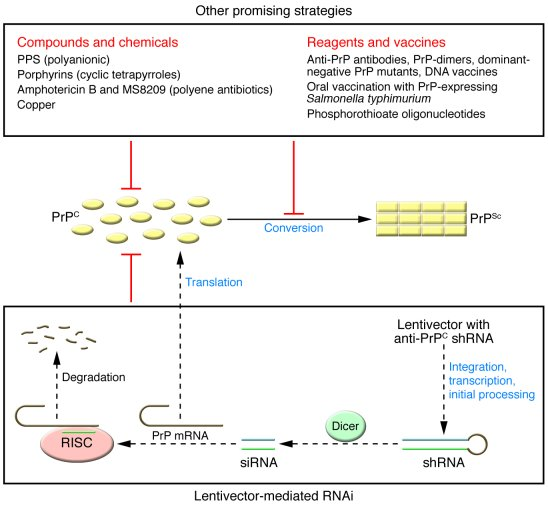
Prion diseases, which include conditions such as Creutzfeldt-Jakob disease, Gerstmann-Sträussler-Scheinker disease, and fatal familial insomnia, are a diverse group of disorders that lead to progressive neurodegeneration. What sets these diseases apart is their unique mechanism of action — they are caused by misfolded prion proteins that induce normal proteins in the brain to also misfold, leading to a cascade of neurological dysfunction. This pathological process highlights how intricate biochemistry underpins prion diseases and the necessity for innovative treatment options that address the root causes rather than just symptoms.
Approximately 15% of prion disease cases are attributed to hereditary mutations in the prion protein gene, while 85% arise sporadically, often with no clear genetic link. Understanding these distinctions is crucial for developing targeted therapies. Additionally, the clinical implications of prion diseases extend beyond individual patients; their infectious nature raises significant public health concerns. Awareness of the types and mechanisms of prion diseases is essential for both researchers and clinicians as they work toward establishing comprehensive treatments and preventive strategies.
The Role of Clinical Trials in Advancing Prion Disease Research and Treatment New therapeutic options for prion diseases rely heavily on the successful transition from research to clinical trials. Clinical trials serve as a critical step in validating the safety and effectiveness of experimental treatments derived from laboratory breakthroughs. The journey from initial discovery to human application involves various phases of trials, often beginning with small-scale studies to ensure safety before moving to larger trials that assess efficacy.
The ongoing research at the Broad Institute provides an excellent example of the rigorous pathway towards clinical trials. By leveraging data obtained from mouse models of prion disease, researchers have established foundational knowledge that will inform trial design. The meticulous nature of these studies not only helps in fine-tuning gene editing therapies but also fosters collaboration among researchers, patient advocates, and clinical specialists, ensuring that the development of new treatments is aligned with the needs of those affected by prion diseases.
Personal Connections: The Drive Behind Prion Disease Research and Treatment As the world grapples with prion diseases, the personal stories of researchers like Sonia Vallabh and Eric Minikel bring urgency and motivation to their scientific endeavors. Vallabh’s diagnosis with fatal familial insomnia — the same condition that claimed her mother’s life — infuses her research with a profound sense of purpose. Their journey, transitioning from established careers in law and planning to focusing on prion diseases, illuminates the power of personal narrative in scientific research.
Moreover, the commitment of patient-scientists in prion disease research fosters a unique synergy, inspiring their colleagues and collaborators. The passion and dedication brought forth by scientists with personal stakes in their work drive progress and innovation, ensuring that research is guided by empathy and the hope of meaningful outcomes. As more individuals share their stories, the collective efforts to combat prion diseases are likely to gain momentum, emphasizing the importance of human experiences in the pursuit of scientific breakthroughs.
Frequently Asked Questions
What recent advancements have been made in prion disease treatment through gene editing therapy?
Recent studies have shown promising advancements in prion disease treatment using gene editing therapy. Researchers at the Broad Institute developed a technique that alters a single base in the gene producing harmful prion proteins, leading to a 50% reduction in these proteins in a mouse model of prion disease. This innovative gene editing approach has significantly extended the lifespan of affected mice, hinting at potential therapeutic options for humans in the future.
How might gene editing therapy be applied to fatal familial insomnia related to prion disease treatment?
Gene editing therapy has the potential to revolutionize fatal familial insomnia treatment by targeting the inherited mutation causing this prion disease. Recent research demonstrates that gene editing can effectively reduce unhealthy prion protein levels, which may offer a new pathway for treating fatal familial insomnia before its symptoms manifest in at-risk individuals.
Are there any ongoing clinical trials related to prion disease treatment using mouse models?
Yes, the innovative techniques developed for prion disease treatment, particularly using mouse models, have laid the groundwork for potential human clinical trials in the coming years. The findings from current mouse studies are essential as they help researchers assess the effectiveness and safety of gene editing therapies before advancing to human subjects.
What key challenges remain before human trials can begin for prion disease treatment?
Several challenges must be navigated before beginning human trials for prion disease treatment. These include refining the delivery mechanism of gene editing therapies, ensuring precision in targeting the specific tissues affected, and enhancing the safety profile of the treatment to minimize any adverse effects associated with vector usage.
Can you explain the role of prion proteins in prion disease and their significance in treatment research?
Prion proteins play a crucial role in the development of prion diseases, as they can misfold and propagate abnormal shapes that cause brain damage and neurodegeneration. Understanding the mechanics of prion proteins is vital for researchers, as developing effective interventions to reduce or eliminate these proteins is central to prion disease treatment strategies.
What collaborative efforts are being observed in current prion disease treatment research?
Current prion disease treatment research showcases strong collaborative efforts among scientists and patient-scientists, particularly between labs focused on gene editing and those specializing in vector engineering. This collaboration enhances the development of innovative treatments by combining expertise, such as that demonstrated by researchers at the Broad Institute, who work closely with patient-advocates to inspire progress and motivation.
What is the significance of the study published in Nature Medicine regarding prion disease treatment?
The recent study published in Nature Medicine holds significant implications for prion disease treatment as it reveals how gene editing can successfully reduce dangerous prion protein levels in a mouse model. This breakthrough serves as a potential catalyst for developing human therapies to combat various prion diseases, sparking hope for future clinical applications.
| Key Point | Details |
|---|---|
| Research Milestone | New gene-editing therapy shows potential for prion disease treatment. |
| Gene Editing Results | Single base change in gene reduced harmful prion protein by half in mice, extending lifespan by 52%. |
| Types of Prion Diseases | Includes Creutzfeldt-Jakob disease, Gerstmann-Sträussler-Scheinker disease, and fatal familial insomnia. |
| Patient Scientist Involvement | Sonia Vallabh, a researcher, tested positive for a hereditary prion disease, driving her research passion. |
| Clinical Trials Timeline | Initial human trials are several years away as many milestones need to be met before then. |
| Collaborative Effort | Combining expertise in technology development and therapeutic application for effective solutions. |
Summary
Prion disease treatment is emerging as a promising area of research, particularly with novel gene-editing therapies showing potential. Advances made by researchers, particularly those with personal ties to the disease, indicate that there may soon be effective treatments available. The initial successes in laboratory experiments involving gene modifications pave the way for future clinical applications that could significantly improve patient outcomes.